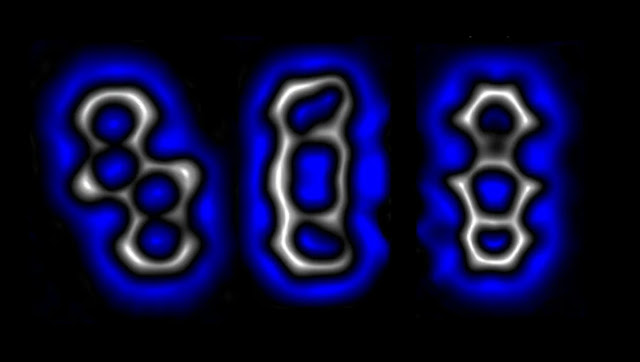
 |
They may look like ghosts but (L to R) bent alkyne,
diradical, and cyclobutadiene are three molecules of the same composition with
different bonds. Image Credit: Leo Gros/IBM |
By adjusting the voltage pulses on an atomic force microscope, chemists have succeeded in rearranging the bonds between atoms in a single molecule.
For chemists, the capacity to change the bonds within
individual molecules, changing the molecule's shape in the process, would be
close to the ultimate power. The capacity to do just that using a (homebuilt)
scanning tunneling microscope and an adjustable voltage has been demonstrated
for the first time, albeit for one rather distinctive molecule.
Building complex molecules is currently a very
inefficient process. In a Perspective in Science, it's described by Professor
Igor Alabugin and Chaowei Hu of Florida State University as like dumping Lego
blocks in a washing machine and hoping they come out in the right combination,
“either by complete chance or under the guidance of other molecular-sized
objects – i.e., catalysts.”
Help may be at hand, however. The same edition of
Science contains the paper Alubugin and Hu are commenting on, which describes a
much more exact process, shaping an atom bond by bond by adjusting the charge
on a tiny copper-tipped probe. Dr Florian Albrecht of IBM Research Europe and co-authors
succeeded in creating three different products starting from the same basic
form, just by choosing different voltage combinations and applying them to
bonds within the molecule. Indeed, they even found the products could be
switched back and forth at will.
The paper describes a process beginning with a
substance called 5,6,11,12-tetrachlorotetracene (C18H8Cl4), from which four
chlorine atoms were removed, leaving behind carbon atoms with at least one
unpaired electron known as radical centers. The radical centers then pair up
creating carbon-carbon bonds but two unpaired electrons.
The system is designed so the unpaired electrons
cannot reconnect to make a further carbon-carbon bond, as would normally occur.
Depending on the voltage applied, the system can maintain its initial form,
known as diradical, or become either cyclobutadiene or bent alkyne. Each of
these have the same composition, but a different arrangement of atoms and
bonding combinations.
“The potential to interact with a different set of
partners makes this shape-shifting molecular system a Swiss Army knife with
three distinct and useful chemical tools,” Alabugin and Hu write. Each is
capable of performing a different chemical function, such as serving as a
binding site for transition metals or participating in redox reactions. The
three could even be used as logic gates in molecular electronics.
Having conducted the experiment at −268° C (−450° F),
a temperature where molecules can be relied on to barely move, replication at
something approaching room temperature may prove more difficult. Other
molecules may also prove harder nuts to crack. Nevertheless, the demonstration
opens the path to precise control of molecular shape in at least some cases.
Moreover, the work will help us understand redox
reactions, which retain plenty of mystery, despite their important role in
organic, and inorganic chemistry.
Reference: Research paper




0 Comments